What Is A Catalytic Converter Bbc Bitesize
Catalytic converters were the answer. Thefts of catalytic converters from cars in England and Wales rose sixfold in 2019.

Scheme Of Work Cambridge Igcse Co Ordinated Sciences Double Award
A catalyst is a substance which changes the rate of reaction but is unchanged at the end of the reaction.
What is a catalytic converter bbc bitesize. This means that the engine uses less fuel and so produces less air pollution. A catalyst is a substance that. Does not alter the products of the reaction.
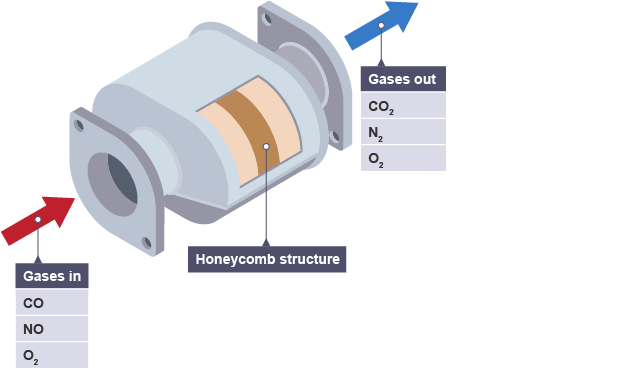
Since the early 1990s car exhausts have been fitted with catalytic converters. Metallic foil monoliths made of Kanthal FeCrAl are used in applications where particularly high heat resistance is required. A diagram of the inside of a car with the catalytic converter highlighted in yellow.
What is a biological catalyst BBC Bitesize. Whats Inside a Catalytic Converter. There has been a huge rise in catalytic converters being stolen from cars in 2019 police in London say.
What is a catalyst Bitesize. It explains why each enzyme will only work on one substrate. Cath causes a lot of reactions - shes a.
A catalytic converter fitted to the exhaust of. Last year there were almost 13000 recorded thefts of the devices with Londoners particularly badly hit. A catalytic converter is an exhaust emission control device that converts toxic gases and pollutants in exhaust gas from an internal combustion engine into less-toxic pollutants by catalyzing a redox reactionCatalytic converters are usually used with internal combustion engines fueled by petrol or diesel including lean-burn engines and sometimes on kerosene heaters and stoves.
For the first six months of 2019 the number of thefts of catalytic converters jumped to. A catalyst is specific to a particular reaction. Catalysts speed up chemical reactions.
Catalytic converter implements to every vehicle drastically lessen the emission of. Speeds up the rate of a reaction. A catalytic converter is a large metal box that sits underneath a car.
These use transition metals such as rhodium palladium and platinum to convert harmful emissions into less harmful substances. Different catalysts catalyse different reactions. An enzyme works on the substrate forming products.
Catalytic converters are used to help to minimize the release of these toxic gases. Reducing harmful emissions today. Catalytic converter thefts double as metal prices rise.
What is a Catalytic Converter. If a catalyst is present the reacting particles can collide more successfully with less energy and so the. Thefts of catalytic converters from motor vehicles have more than doubled over the past three years a BBC.
In this article you can read what is a catalytic converter and why you need it. A catalyst is a substance which changes the rate of reaction but is unchanged at the end of the reaction. They help reduce the chances of us getting ill by reducing the emission of toxic fumes.
Inside theres typically a piece of ceramic coated with various precious metals including palladium rhodium and platinum which are the catalysts that create a chemical reaction to convert the harmful gases into safer substances. For automotive catalytic converters the core is usually a ceramic monolith with a honeycomb structure. Different catalysts catalyse different reactions.
Likewise people ask what are catalysts BBC Bitesize. Engineers are developing engines for vehicles that are more efficient. A catalyst speeds up the rate of a reaction but it is not used up in the reaction.
Catalytic converters are used with internal combustion engines fuelled by either petrol or diesel. Only a very small amount of catalyst is needed to increase the rate of reaction between large amounts of reactants. Learn about and revise the Earths atmosphere with this BBC Bitesize GCSE Chemistry OCR 21C study guide.
A catalyst is specific to a particular reaction. Enzymes are proteins that act as biological catalysts - this means they speed up reactions without being used up. Is not chemically changed or used up at the end of the reaction.
An enzymes active site and its substrate are complementary in shape. Mags explains how catalysts work at a visit to the funfair with cousin Cath. Only a very small amount of catalyst is needed to increase the rate of reaction between large amounts of reactants.

A Flow Diagram Summarising The Manufacture Of Ammonia From Natural Gas Methane Steam And Air Chemical Engineering Nursing Student Tips Business Education

Gcse Science Revision Chemistry Catalysts Youtube

Kinetics Theory Of Catalytic Mechanisms Heterogeneous Catalysis Homogeneous Catalyzed Reaction Examples Advanced A Level Gce Revision Notes

Reducing Pollutants Mapa Mental

Air Cie Igcse Chemistry Revision Notes

Ammonia Ammonia Physical Science Diagram

Was Your Catalytic Converter Stolen Here S Why Reactions Science Videos American Chemical Society

Catalytic Converter Catalysts Gcse Chemistry 9 1 Kayscience Com Youtube

Revision Checklist For Igcse Chemistry 0620

Kinetics Theory Of Catalytic Mechanisms Heterogeneous Catalysis Homogeneous Catalyzed Reaction Examples Advanced A Level Gce Revision Notes

Temperature Concentration Graphs Gcse Chemistry Reaction Rate Science Revision

Air Cie Igcse Chemistry Revision Notes

Flowsheet Chemical Engineering Chemistry Infographic

Look4chemistry Reaction Rate Surface Surface Area

What Are Catalytic Converters Environment Chemistry Fuseschool Youtube

Air Cie Igcse Chemistry Revision Notes

Bbc Top Gear Magazine Issue 08 2021
Eli5 How Does A Catalytic Converter Reduce Emissions Explainlikeimfive

Post a Comment for "What Is A Catalytic Converter Bbc Bitesize"